CENTRAL STERILE SUPPLY DEPARTMENT: Planning Considerations
A hospital consumes a large quantity of new material that needs sterilization before use. It also processes other material that has to be cleaned and sterilized before it can be used again. Central Sterile Supply Department (CSSD) is a service whereby medical/surgical supplies and equipment – both sterile and nonsterile – are cleaned, prepared, processed, stored and issued for patient care. Hospital acquired infection remains a serious problem in health care today. The purpose of a sterile services department is to concentrate the skill and the responsibility for the supply of sterile material and to reduce the risk of error.
The primary activities to be undertaken within the CSSD are:
1. Cleaning and disinfecting processes for instruments, trays, utensils, containers and other reprocessable items.
2. Preparing and packaging contents of trays and packs and where appropriate, single-use items and other materials as supplementary packs.
3. Sterilizing trays and packs and disinfecting those items acceptable for patient use in this condition.
4. Storing non-sterile materials components.
5. Storing goods processed in the department and purchased sterile goods.
6. Distributing processed and purchased goods to users.
Sterilization of instruments, operating packs, trays etc., is performed is performed by heating them with pressurized steam or by gas sterilization. Steam sterilization is called autoclaving. However, certain items such as rubber, plastic and delicate instruments cannot be autoclaved and so have to be sterilized by using ethylene oxide or similar gases. Gas sterilization requires certain safety precautions such as aeration prior to use and special exhaust ventilation. Under both systems, sterilization is performed on cleaned instruments wrapped in special linen.
The department receives clean material from a laundry and new material from manufacturers and suppliers. It also receives for re-use, dirty articles from within the hospital. Clean and dirty materials require separate delivery points, the clean one serving a bulk store for new materials such as towels and masks, and the dirty one serving a clean-up room where all re-usable goods including instruments and syringes are washed, cleaned and dried. Rubber gloves may require a separate glove room for treatment.
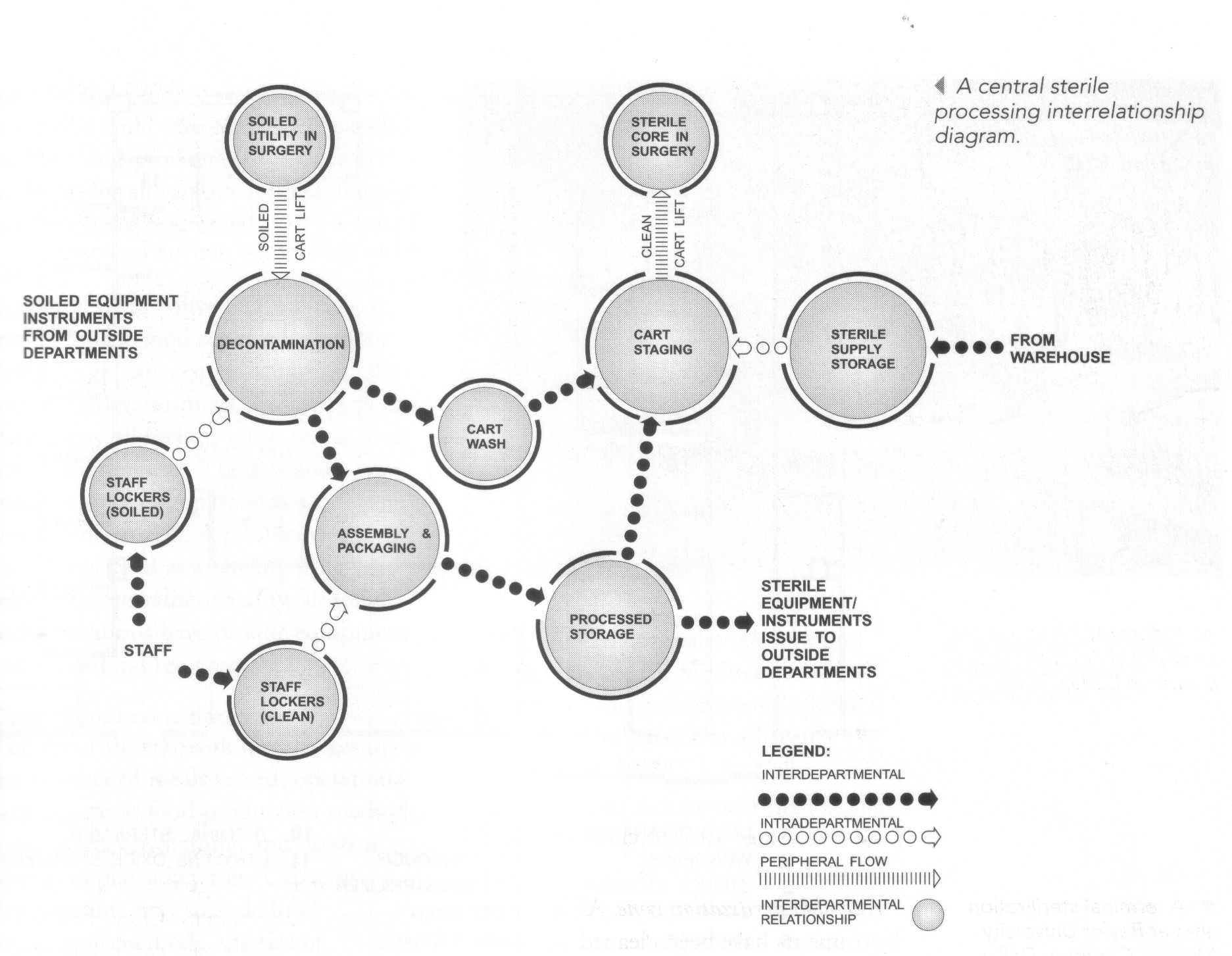
The department is divided into three zones to accomplish the functions of decontamination, assembly and sterile processing, and sterile storage and distribution. These zones include the following:
1. Decontamination zone
2. Assembly/sterilization zone
3. Storage and distribution zone
The work flow for the central sterile supply department is centered on the processing of soiled instruments through the four zones. A distinct separation must be maintained between the soiled and sterile areas. The technical staff works on either the soiled side or the sterile side and cannot cross from one side to the other.

A decontamination area at Baylor University Medical Center in Dallas, Texas
Decontamination zone. Reusable equipment and soiled instruments and supplies are received from surgery, labor/delivery and other departmental areas for initial or gross cleaning. These items are cleaned and decontaminated by means of manual or mechanical processes and chemical disinfection. The exchange cart is cleaned in a pass-through cart washer and readied in the assembly zone to carry items back to the departments. Items of equipment used in this area include the following:
Biohazardous waste management systems
Washer/Decontaminator – used to clean heat-tolerant items
Ultrasonic Washer – used to remove fine soil from surgical instruments after manual cleaning and before sterilization
Healthcare decontamination systems (pass-through washer sterilizers or tunnel washers) – used to sterilize instruments in perforated or mesh-bottom trays
Cart washers – used to clean carts and other transport vehicles
Assembly/sterilization zone. After the instruments have been cleaned and inspected, they are typically assembled into sets or trays, according to detailed instructions. Each set or tray is wrapped or packed in a non-woven textile pouch or a rigid package/container system for terminal, or final, sterilization. At that point, the sets are prepared for issue, storage or further processing. After assembly, the instruments receive final sterilization. The cleaned instruments are issued to the sterile storage area until issued. Equipment used most commonly in this zone includes the following:
High-pressure sterile processing systems (steam or electric)
Low-pressure sterile processing systems
ETO (Ethylene Oxide) gas sterilizer and aerators
ETO gas aerators
Chemical sterilization systems
Microwave sterilization systems

A terminal sterilization area at Baylor University Medical center in Dallas, Texas
Storage and distribution zone. Following the sterilization process the instruments are stored in sterile storage or sent to the appropriate department. Other functions of this zone include case cart preparation and delivery; telephone or requisition order filling, and delivery of patient care equipment.
It is advisable to have one high-speed autoclave, preferably in the surgical suite, as a standby in the event of a CSSD breakdown. Flash sterilization is performed in the user departments, particularly the operating rooms, to resterilize the instruments needed immediately or those that have been dropped accidentally. Flash sterilization is autoclaving an instrument when it is unwrapped.
The department should be in the hospital service zone to simplify the reception of goods. Proximity to the boiler room is an advantage if steam is raised there. Good communication routes to most of the other departments of the hospital are essential. However, it has a relationship primarily with the Surgical Suite, and can be placed next to it. It can also be located above or below the Surgical Suite. This requires elevators or dumbwaiters to provide direct access for both clean and soiled materials to and from surgery. In some facilities, the central sterile supply department is collocated with materials management.
The size of the central sterile supply department area depends on the number of surgical and obstetrics cases treated in a given period and the amount (cubic volume) of sterile storage required. In addition the number of open heart and/or orthopedic cases treated in a given period must be considered. Key capacity determinants include the number and type of sterilization instruments, the exchange case cart distribution system, and instrument holding and equipment cleaning in the CSSD department.
The trend is for the central sterile supply department to move into total integration with surgery. This move is in response to physicians’ continued concern regarding the handling of surgical instruments and the need for nurses to prepare the case trays for sterilization.
Material used in this article may have been sourced from some or all of the following books. We strongly urge you to purchase those that are of interest to you in your pursuit of knowledge in the field of Healthcare Facility Planning and Design.
American Institute of Architects Academy of Architecture for Health with assistance from the U.S. Department of Health and Human Services, Guidelines for Design and Construction of Hospital and Health Care Facilities. Washington D.C.: The American Institute of Architects Press, 1996.
Cox, Anthony and Groves, Philip, Hospitals and Health-Care Facilities: A Design and Development Guide. London : Butterworth Architecture, 1990.
Hain, Walter, Laboratories: A Briefing and Design Guide. London : Chapman & Hall, 1995.
Kliment, Stephen A., Building Types Basics for Healthcare Facilities. New York : John Wiley & Sons Inc., 2000
Kunders,G.D., Gopinath S., and Katakam A., Hospitals : Planning, Design and Management. New Delhi : Tata McGraw-Hill, 1998.
Miller, Richard L. and Swensson, Earl S., New Directions in Hospital and Healthcare Facility Design. New York : McGraw-Hill, Inc., 1995.
Putsep, Ervin, Modern Hospital. London : Lloyd-Luke Ltd., 1981.
Smith, Judith A., The Family Birthplace: Planning and Designing Today’s Obstetric Facilities. USA : American Hospital Publishing Inc., 1995.
Central Sterile Supply Services
Sterile Processing
Behind the Scenes: Sterile Processing Department
Sterile Processing Technician–Donna Reich
3 thoughts on “CENTRAL STERILE SUPPLY DEPARTMENT: Planning Considerations”